What is Resonance structure ? Why the resonances stricture required explain with suitable example.
What is Resonance structure ? Why the resonances stricture required explain with suitable example.
Resonance Structures : Different Lewis structure of single molecule / ion is known as resonance structure. According to the concept of resonance, whenever a single Lewis structure cannot describe a molecule accurately so more than one Lewis structure means resonance structure describe.
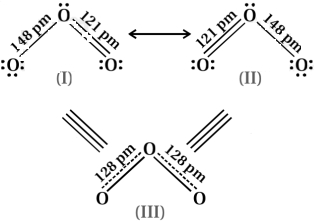
Resonance structure of ozone $\left(\mathrm{O}_{3}\right)$ : To draw resonance structure the position of nucleus of atom is not change. Every reasons structure the position of bonding and non-bonding electron is change but Lewis structure is not seen different. The different resonance structure is represented by a double headed arrow. All resonance structure are similar energy.
Two resonance structure of $\mathrm{O}_{3}$ molecule $(I)$ and $(II)$ and $(III)$ is resonance hybrid.
Limitation of resonance : Any one is not seen right structure. The $\mathrm{O}-\mathrm{O}$ bond length is 148 and $\mathrm{O}=\mathrm{O}$ bond length is $121 \mathrm{pm}$. Thus single and double bond length is not experimentally present in $\mathrm{O}_{3}$.
$\therefore$ Correct structure of $\mathrm{O}_{3}$ is not $(I)$ and $(II)$
Resonance : Correct structure is resonance hybrid. Experimentally determined oxygen - oxygen $(\mathrm{O}-\mathrm{O})$ bond length in the $\mathrm{O}_{3}$ molecule are same $128 \mathrm{pm}$. So, $(III)$ is a real or accurate structure of $\mathrm{O}_{3}$ in which single and double bond is not stable. Resonance structure are necessary because it gives correct different Lewis structure.
Orientation : Single assumption structure can't give correct bond length \& bond energy.
Advantage : It gives assumption of correct structure.
Similar Questions
How many resonance forms can be written for the nitrate ion, $(NO^-_3)$ ?
How many resonance forms can be written for the nitrate ion, $(NO^-_3)$ ?
Write the resonance structure for ${\rm{S}}{{\rm{O}}_3},{\rm{N}}{{\rm{O}}_2}$, and ${\rm{NO}}_3^-$.
Write the resonance structure for ${\rm{S}}{{\rm{O}}_3},{\rm{N}}{{\rm{O}}_2}$, and ${\rm{NO}}_3^-$.
Bond length of ethane $(I),$ ethene $(II),$ acetylene $(III)$ and benzene $(IV)$ follows the order
Bond length of ethane $(I),$ ethene $(II),$ acetylene $(III)$ and benzene $(IV)$ follows the order
How many $\sigma $ and $\pi $ bonds are there in the molecule of tetracyanoethylene
$\begin{array}{*{20}{c}} {N \equiv C} \\ {N \equiv C}\end{array}\,\,\left. {} \right\rangle C = C\,\left\langle {} \right.\,\begin{array}{*{20}{c}} {C \equiv N} \\ {C \equiv N} \end{array}$
How many $\sigma $ and $\pi $ bonds are there in the molecule of tetracyanoethylene
$\begin{array}{*{20}{c}} {N \equiv C} \\ {N \equiv C}\end{array}\,\,\left. {} \right\rangle C = C\,\left\langle {} \right.\,\begin{array}{*{20}{c}} {C \equiv N} \\ {C \equiv N} \end{array}$
$CO_3^{2 - }$ anion has which of the following characteristics
$CO_3^{2 - }$ anion has which of the following characteristics


