Two substances $A$ and $B$ of equal mass m are heated at uniform rate of $6\, cal s^{-1}$ under similar conditions. $A$ graph between temperature and time is shown in figure. Ratio of heat absorbed ${H_A}/{H_B}$ by them for complete fusion is
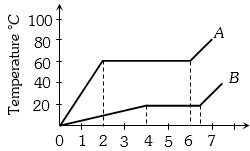
Two substances $A$ and $B$ of equal mass m are heated at uniform rate of $6\, cal s^{-1}$ under similar conditions. $A$ graph between temperature and time is shown in figure. Ratio of heat absorbed ${H_A}/{H_B}$ by them for complete fusion is

- A
$\frac{9}{4}$
- B
$\frac{4}{9}$
- C
$\frac{8}{5}$
- D
$\frac{5}{8}$
Similar Questions
A thermally insulted vessel contains $150\, g$ of water at $0\,^oC$. Then the air from the vessel is pumped out a adiabatically. A fraction of water turns into ice and the rest evaporates at $0\,^oC$ itself. The mass of evaporated water will be closes to ....... $g$ (Latent heat of vaporization of water $= 2.10 \times10^6\, Jkg^{-1}$ and Laten heat of Fusion of water $ = 3.36 \times10^5\,Jkg^{-1}$ )
A thermally insulted vessel contains $150\, g$ of water at $0\,^oC$. Then the air from the vessel is pumped out a adiabatically. A fraction of water turns into ice and the rest evaporates at $0\,^oC$ itself. The mass of evaporated water will be closes to ....... $g$ (Latent heat of vaporization of water $= 2.10 \times10^6\, Jkg^{-1}$ and Laten heat of Fusion of water $ = 3.36 \times10^5\,Jkg^{-1}$ )
- [JEE MAIN 2019]
A block of ice at $-10^{\circ} \mathrm{C}$ is slowly heated and converted to steam at $100^{\circ} \mathrm{C}$. Which of the following curves represent the phenomenon qualitatively:
- [JEE MAIN 2024]
What are the states of matter ? Which ?
What are the states of matter ? Which ?
The point on the pressure temperature phase diagram where all the phases co-exist is called
The point on the pressure temperature phase diagram where all the phases co-exist is called
A substance is cooled at a constant power. Its temperature vs time graph is shown. The value of $S_{solid} : S_{liquid} : S_{gas}$
A substance is cooled at a constant power. Its temperature vs time graph is shown. The value of $S_{solid} : S_{liquid} : S_{gas}$
