Give resonance of ${{\rm{O}}_3}$, And Explain its requirement, delimitation and advantages
Give resonance of ${{\rm{O}}_3}$, And Explain its requirement, delimitation and advantages
Resonance Structures : Different Lewis structure of single molecule / ion is known as resonance structure. According to the concept of resonance, whenever a single Lewis structure cannot describe a molecule accurately so more than one Lewis structure means resonance structure describe.
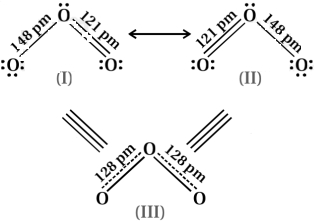
Resonance structure of ozone $\left(\mathrm{O}_{3}\right)$ : To draw resonance structure the position of nucleus of atom is not change. Every reasons structure the position of bonding and non-bonding electron is change but Lewis structure is not seen different. The different resonance structure is represented by a double headed arrow. All resonance structure are similar energy.
Two resonance structure of $\mathrm{O}_{3}$ molecule $(I)$ and $(II)$ and $(III)$ is resonance hybrid.
Limitation of resonance : Any one is not seen right structure. The $\mathrm{O}-\mathrm{O}$ bond length is 148 and $\mathrm{O}=\mathrm{O}$ bond length is $121 \mathrm{pm}$. Thus single and double bond length is not experimentally present in $\mathrm{O}_{3}$.
$\therefore$ Correct structure of $\mathrm{O}_{3}$ is not $(I)$ and $(II)$
Resonance : Correct structure is resonance hybrid. Experimentally determined oxygen - oxygen $(\mathrm{O}-\mathrm{O})$ bond length in the $\mathrm{O}_{3}$ molecule are same $128 \mathrm{pm}$. So, $(III)$ is a real or accurate structure of $\mathrm{O}_{3}$ in which single and double bond is not stable. Resonance structure are necessary because it gives correct different Lewis structure.
Orientation : Single assumption structure can't give correct bond length \& bond energy.
Advantage : It gives assumption of correct structure.
Similar Questions
Choose the $CORRECT$ comparison of decreasing $\pi -$ bond order of $S-O$ bond ?
Choose the $CORRECT$ comparison of decreasing $\pi -$ bond order of $S-O$ bond ?
The number of possible resonance structures for $CO_3^{2 - }$is
The number of possible resonance structures for $CO_3^{2 - }$is
Which of the following conditions is not correct for resonating structures?
Which of the following conditions is not correct for resonating structures?
Resonating structures have different
Resonating structures have different
Bond length of ethane $(I),$ ethene $(II),$ acetylene $(III)$ and benzene $(IV)$ follows the order
Bond length of ethane $(I),$ ethene $(II),$ acetylene $(III)$ and benzene $(IV)$ follows the order


